The timely application of zinc can prevent yield losses farmers must make sure that their soil I healthy as ha an optimum chemical status before a crop I planted. Leaf, plant and soil analyses for micronutrients are an excellent aid for long-term planning.
Soil fertility, the soil’s potential to supply the growing plant with nutrients is determined by the availability of nutrient reserves in the soil, factors that ill influences their availability and other growth limiting actors. Fertility can be manipulated. Farmers can deplete soil reserves through over-cropping (continuous cropping without fertiliser applications) or they can maintain and improve it by fertilisation, liming and optimal cultivation and moisture conservation methods. Farmers must at all ties apply sufficient macro- and micronutrients to utilise the soil’s production potential and the yield potential of the crop.
This will include a liming programme to maintain an optimum pH level maintaining the carbon content (organic material) is as important.
According to the Fertiliser Handbook of the Fertiliser Society of South Africa (FSSA), farmers just use the potential of the soil and crop in determining their fertiliser programmes. The climatic conditions will also play a role in this decision-making process. Soil types vary in suitability for different crops. Soil classification is an aid in planning a crop production system, but the soil’s chemical composition, nutrient status, depth, texture rainfall and field properties are all important.
Once the farmer has decided on the crop, the fertiliser programme can be planned after ascertaining the chemical and nutrient status of the soil. Soils are analysed to establish if any corrective measures are needed macro- or micronutrient imbalances or deficiencies present that may influence plant growth must be ascertained.
To establish the nutrient needs for a specific crop yield, the soil mineral contribution must be taken into account and compared with the plant’s needs it will also depend on how much of a specific element the crop will remove from the soil reserves.
Mr Fritz Otto from Zinchem, a local supplier of zinc for agricultural use, says that clay soils have a high exchange capacity (the potential of the soil to attract and hold soluble elements) and will have more zinc than soils low in clay. The farmer must know the zinc level in the soil and how much the crop needs for optimal production. All soils have a minimum zinc maintenance level for microbial activity and plant growth.
According to information supplied by a fertiliser company, zinc has the following functions and is influenced in the following ways:
- It is an important component of organic acids;
- Copper suppresses the uptake of zinc;
- High phosphate levels suppress zinc uptake;
- It plays an important role in protein synthesis;
- Zinc deficiencies reduce the concentration of tryptophan in maize and reduce indole-acetic acid and auxsins (a growth hormone);
- Zinc play an important role in starch production and grain filling;
- Cold, wet conditions reduce zinc uptake.
Mr Tem Odendaal from Soygro, a manufacturer of biological inoculants, uses worldwide research data to ascertain average nutrient removal figures for crops. The table below gives the values for micronutrients.
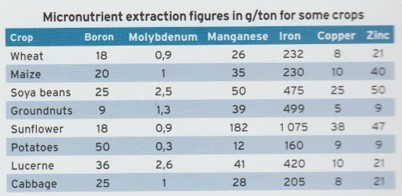
The values differ from crop to crap as yields and withdrawal from the soil varies. These are approximate values and can vary within a crop and between cultivars.
Mr Andre Immelman had agreed that the data supports international research; however, farmers should not use the data as a reason not to apply zinc. The information does not take into account the zinc source (sulphate or oxide) and the level of leaching of zinc in the soil. If farmers are of the opinion that 0.5% zinc in NPK mixtures (nitrogen, phosphorus and potassium) is enough and ore zinc isn’t necessary, misunderstandings may occur.
Immelman explained: according to the table, a maize yield of 1 ton per hectare will remove 40g zinc. A maize yield of 5 tons per hectare will thus extract 200g zinc per annum from the soil (5 x 40g). a farmer who fertilisers with 400kg of NPK with 0.5% zinc will add 2kg zinc to the soil (400kg x 0.5% Zn). This means that a farmer applies 10 times more zinc than removed by the crop. The question is: is there any reason to apply more zinc, as the 0.5% zinc in the NPK mixture supplies enough and even more than what is needed? Why was it necessary to add 0.5% zinc to the NP mixture? And why is it that in South Africa the zinc level in maize are still so low?
Zinc oxide has been used as a zinc source in fertiliser for years because it was thought to be cheaper than zinc sulphate or chelate. However, zinc oxide is not water soluble like zinc sulphate. Zinc oxide is therefore a less efficient source of zinc for plants. Unused zinc oxide in the soil will be leached over a period. There is conflicting international research data with regard to the leaching of zinc oxide. Unfortunately, the general perception is that zinc oxide is a ‘slow-release’ source of zinc and does not leach.
The solubility of zinc is influenced by the pH of the soil and zinc is insoluble at a high ph.
There is an increasing awareness in the fertiliser industry that zinc concentrations in fertilisers should be increased. The form of the zinc is more important than the level in fertilisers. Di Johan van Biljon, a well-known plant nutritionist, reported in the FSSA journal that zinc should be applied when the soil level is below 3.8mg/kg (standard o.1M hydrochloric acid extraction method). Previously the FSSA worked on 2mg/kg soil level. Immelman stated that Zinchem accepted critical soil zinc levels of 6mg/kg for sandy soils and 9mg/kg for clay soils. These values are supported by the Albrecht system.
Zinc oxide is not water soluble like zinc sulphate. Zinc oxide is therefore a less efficient source of zinc for plants.
Van Biljon says that little research on zinc was done during the pas half century, since it was first observed in the late fifties and early sixties. Since then zinc was applied at a rate of 50g/hectare and up to 2kg/hectare at planting. When soil levels are below 1.5mg/kg to 2mg/kg, set guidelines are currently used for zinc application. However, he strongly recommends that at threshold value of 3.8mg/kg zinc must be applied for optimum yield levels.
Large maize production areas are low in zinc. The perception that zinc deficiencies are something of the past is not true.
Van Biljon reports that a study financed by the Maize Trust done by the SA Grain Laboratory found that the average zinc content of maize during the 2003/4 season was 12.35mg/kg and during the 2004/5 season was 13.47mg/kg. this gave rise to maize meal supplemented with 18.55mg zinc per kilogram in accordance with Government Gazette regulations.
Dr Willem Albrecht, who developed the much-debated Albrecht system, says that farmers must return to basic soil science principles. Using accurate soil analytical data, he studied the influence and recovery of acro- and micronutrients and humus in soil. He says that this approach is the key to sustainable and profitable biological and organic farming.
This will create the ideal environment for soil organisms to supply the needs of the growing plant. In principle, the system uses the soil’s chemical and physical properties to create an optimal biological environment.
Immelman said the Albrecht system has, fir five decades, been at the forefront of research on micronutrients and the relationship between macronutrients and micronutrients according to Otto, the Albrecht system has supported the importance of zinc for the last 60 years. The system focuses on the importance of micronutrients and nutrient relationships. Mineralisation of soil and the correct application of micronutrients will reduce the need for macronutrient products. International research supports this principle.
Immelman said that an analysis by Zinchem of thousands of soil samples for the presence of zinc shows that many South African soils contain less than 3.8ppm of zinc (ppm is the same as g/kg) and to a greater degree, less than 2ppm.
A practical way to calculate the amount of zinc sulphate needed to increase the zinc level is the following. Each 10kg monohydrate zinc sulphate per hectare will increase the soil zinc by 1mg/kg. to increase a soil zinc analysis form 2mg/kg to 6mg/kg, 40kg of zinc sulphate per hectare must be applied. This is true for broadcast applications. In the case of band placement smaller quantities will be needed. If the pH is not optimal the applied zinc will be less available.
Standard fertiliser practices alone will not give the best results; they must be supplemented by water soluble micronutrients.
It is important to ensure tat there is an adequate amount of all micronutrients throughout the season timely corrections are important. Leaf, sap and plant analyses can be done.
Farmers must not wait until deficiency symptoms occur as yields will be affected. Timely foliar sprays are the best way to prevent potential losses. Leaf, plant and soil analyses for micronutrients can assist with long-term production planning.
It is important to ensure that there is an adequate amount of all micronutrients throughout the season.
In planning the next season’s requirements, foliar sprays are a short-term solution, says Otto, as plants do not feed through their leaves.
This is effective in solving deficiencies when they develop, but nutrition via the roots is vital for high yields.
Mr Sudesh Singh, sales and marketing manager of Zinchem, classifies crops according to their zinc requirement in the following groups:
- High-demand: applies avocados green beans, groundnuts, guavas, macadamias, mangos, maize, oranges pecan nuts, pears bananas, pineapples, sunflower, lemons and onions;
- High to medium demand: sorghum and tomatoes;
- Medium demand: strawberries, potatoes, grapes, canola, cherries, cucumbers, cabbage, pumpkins, soya beans, sugar cane and tobacco;
- Medium to low demand: wheat;
- Low demand: Rooibos tea.
Immelman is not prepared to guarantee higher yields but points out that numerous experiments worldwide over the past 20 years have shown increased yields with zinc sulphate.
With even small applications, yield increases of 10%, 20%, 50% and even 100% were recorded. The key question is, to what level has the soil’s zinc level dropped below the critical value? The lower the zinc level, the higher the potential yield increase.
In a field trial with groundnuts in the Free State, a farmer increased yields by 40%, with an increased bean grading, this resulted in higher grading class and in the total ton per hectare. In such situation’s farmers can increase their profits 10 to 15 times the cost of the zinc sulphate.
Different soil types have differing effects on the ability of the plant to adsorb zinc. Otto says it will depend on the clay colloid’s ability to adsorb or release zinc.
Sandy soil has a low clay content and can adsorb only very small quantities of zinc. The type of clay will also play a role.
Some clay soils have a higher adsorption capacity for minerals than others. Cation nutrients have a different order of adsorption on the clay. It is generally accepted that minerals such as zinc, manganese and copper in soil are adsorbed on organic molecules such as humus.
How do foliar sprays compare with soil applications?
Otto says that both are important in high level production systems.
The one cannot go without the other. Focus on the bigger picture of soil maintenance rather than on only plant or only soil needs as a mineral balance supports the biodiversity of soil ecology. However, micronutrients are highly available for plants through their leaves.
Original article by Charl van Rooyen
